The BATTLE to Personalize Lung Cancer Prevention through Reverse Migration
Kathryn A. Gold1, Edward S. Kim1, J. Jack Lee2, Ignacio I. Wistuba1,3, Carol J. Farhangfar4, and Waun Ki Hong1,4
+ Author Affiliations
Authors' Affiliations: Departments of 1Thoracic/Head and Neck Medical Oncology, 2Biostatistics, and 3Pathology, and 4Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center, Houston, Texas
Corresponding Author:
Waun Ki Hong, Division of Cancer Medicine, Unit 421, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030. Phone: 713-792-7770; Fax: 713-404-9696; E-mail: whong@mdanderson.org.
Abstract
Agents can enter clinical development for cancer prevention either initially or after previous development for a different indication, such as arthritis, with both approaches consuming many years of development before an agent is fully evaluated for cancer prevention. We propose the following, third approach: reverse migration, that is, importing agents, targets, and study designs to personalize interventions and concepts developed in advanced cancer to the setting of cancer prevention. Importing these “ready-made” features from therapy will allow reverse migration to streamline preventive agent development. We recently reported the Biomarker-integrated Approaches of Targeted Therapy for Lung Cancer Elimination (BATTLE) trial of personalized lung cancer therapy and now propose the reverse migration development of personalized lung cancer prevention based on the BATTLE model. Cancer Prev Res; 4(7); 962–72. ©2011 AACR.
Table 1. The reverse migration of tamoxifen
| Treatment setting
| Results |
Tamoxifen vs. DES, 1981 (114) | Metastatic diseasea | Response rate 33% on tamoxifen monotherapy |
EBCTCG meta-analysis, 1998 (115) | Adjuvant treatment | 47% decrease in recurrence with 5 y of tamoxifen vs. placebo |
B-24, 1999 (116) | DCIS | 43% decrease in IBC, 32% decrease in NIBC with tamoxifen vs. placebo |
BCPT, 1998 (5) | Healthy, moderate-risk women | 49% decrease in IBC, 40% decrease in NIBC with tamoxifen vs. placebo |
Abbreviations: BCPT, Breast Cancer Prevention Trial; DES, diethylstilbestrol; DCIS, ductal carcinoma in situ; EBCTCG, Early Breast Cancer Trialists' Collaborative Group; IBC, invasive breast cancer; NIBC, noninvasive breast cancer.
aHormone receptor status was not measured prior to enrollment on trial.
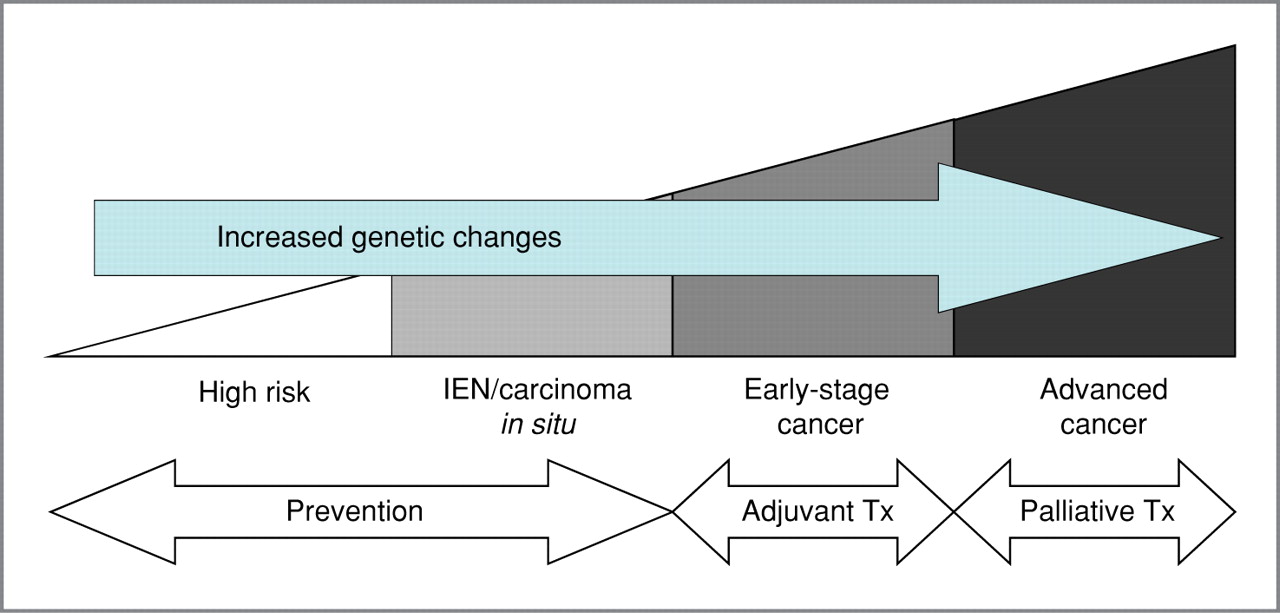
Figure 1.
Schema showing progression from high-risk states to advanced lung cancer, correlating with increased genetic changes, and the treatment (Tx) options at each stage. Intraepithelial neoplasia (IEN) includes hyperplasia, metaplasia, and dysplasia.
Table 2.
Relevant randomized controlled trials in lung cancer chemoprevention highlighting differences between current and former smokers
Trial | Population | Intervention | Endpoint | Outcome—current smokers | Outcome—former smokers |
ATBC, 1994 (18) | Male current smokers | α-Tocopherol, β-carotene | Lung cancer | Increase in lung cancer | N/A |
CARET, Omenn et al., 1996 (32) | Smokers (current or former) | β-Carotene, retinol palmitate | Lung cancer | Increase in lung cancer | Trend toward decrease in lung cancer |
Lippman et al., 2001 (34) | Following resection of stage I NSCLC | 13-cis-RA | Survival | Decreased survival | Trend toward increased survivala |
Kurie et al., 2003 (43), Hittelman et al., 2007 (117) | Former smokers | 9-cis-RA or 13-cis-RA plus α-tocopherol | RAR-β, Ki-67, and metaplasia | N/A | Increase in RAR-β and decrease in Ki-67 |
Mao et al., 2011 (45) | Former smokers | Celecoxib | Ki-67 labeling index | N/A | Decrease in Ki-67 |
Keith et al., 2011 (47) | Smokers (current or former) | Oral iloprost | Endobronchial dysplasia | No histologic improvement | Improvement in histology |
Abbreviations: 9-cis-RA, 9-cis-retinoic acid; 13-cis-RA ATBC, 13-cis-retinoic acid; Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; CARET, Carotene and Retinol Efficacy Trial; RAR-β, retinoic acid receptor-β.
aNonsmokers enrolled on this trial had a trend toward lower death rate with 13-cis-RA vs. placebo: 2% vs. 5.2%, P = 0.14.

Figure 2.
Schema showing some of the different histopathologic and molecular pathways involved in lung carcinogenesis. NF-κB, nuclear factor kappa B; TSG, tumor suppressor gene.
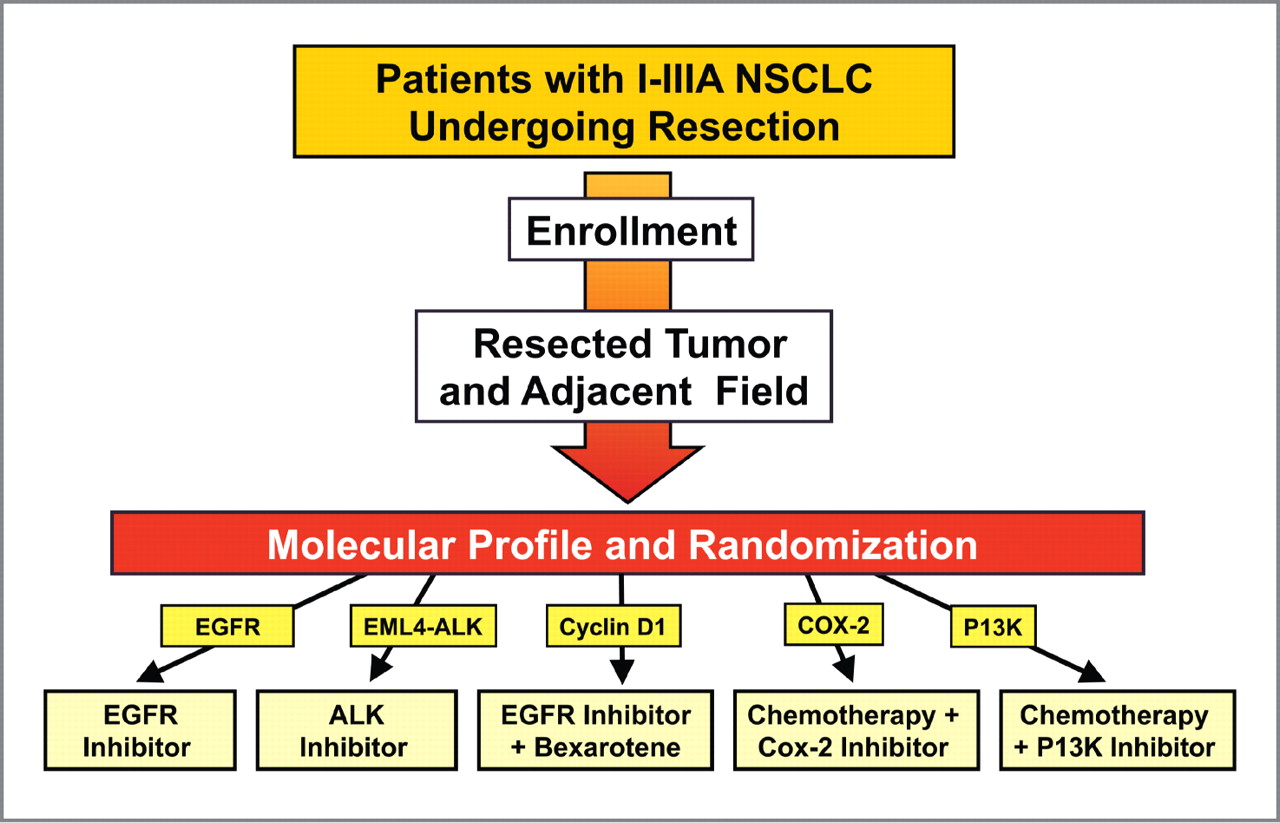
Figure 3.
Schema for a personalized trial of tertiary chemoprevention/adjuvant treatment. The tumor and adjacent field will undergo biomarker analysis, and patients will be grouped based on marker status. In the EGFR group, patients with EGFR mutations will receive EGFR inhibitors. Patients with EML4-ALK translocations by FISH will receive an inhibitor of the fusion protein such as crizotinib. The cyclin D1 group will include patients with cyclin D1 overexpression. Patients with a high ratio of COX-2 to 15-PGDH or high expression of COX-2 will receive a COX-2 inhibitor in combination with chemotherapy. The PI3K group will include patients with either PI3K amplifications or mutations or PTEN loss.
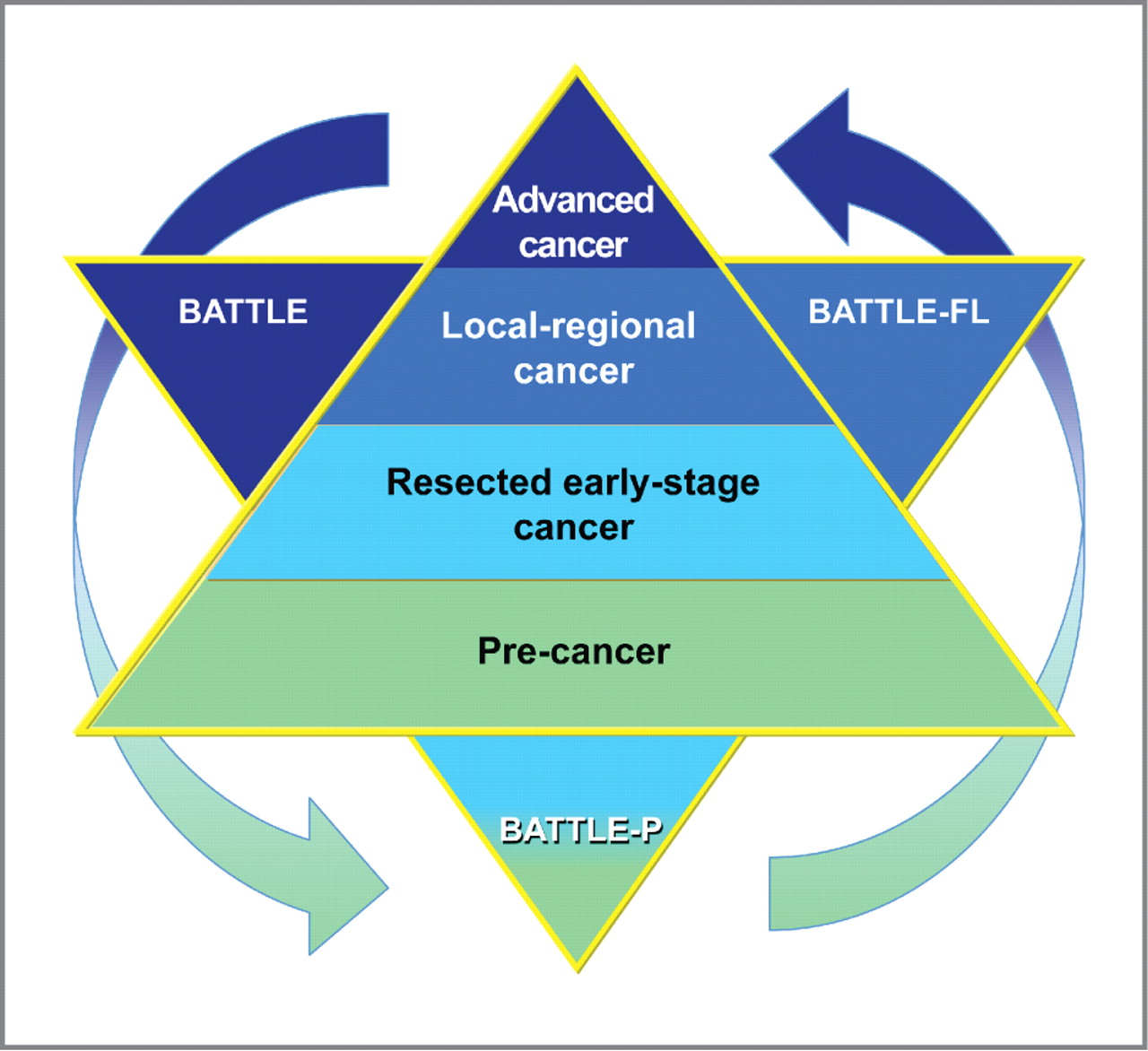
Figure 4.
Schema for a comprehensive BATTLE strategy against all stages of carcinogenesis, from advanced malignancy to premalignancy (precancer). BATTLE (top left) is our completed trial in advanced cancer. BATTLE-Frontline (BATTLE-FL, top right) is our developing trial in locoregional disease. BATTLE-P (bottom) comprises the adjuvant (resected, early-stage cancer) and premalignancy/precancer settings.



